Clinical Trials – Enrollment
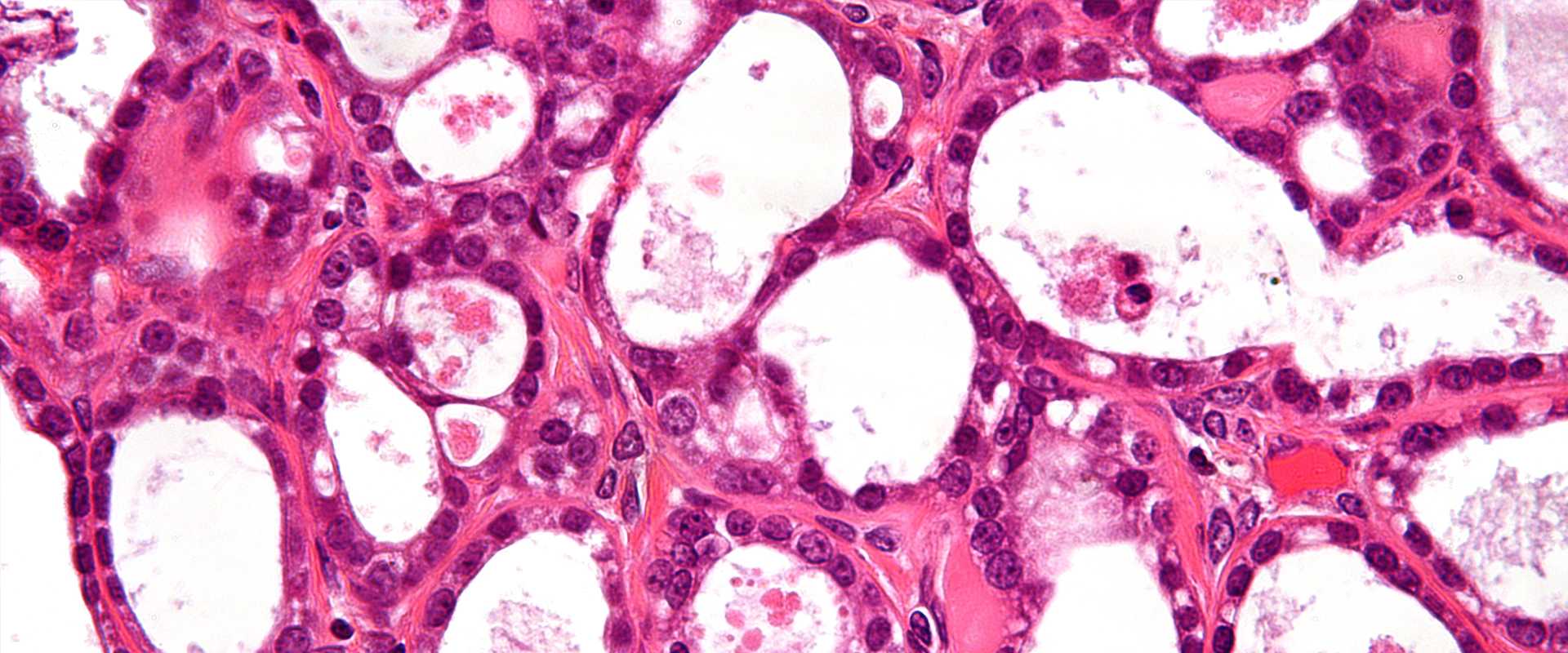
Ovarian Cancer clinical trial.
Complete the form to connect with CSSIFM and start your clinical trial journey
Trial enrollment process
The Enrollment Process: What to Expect
- Initial Online Questionnaire
- Complete our brief online form so we can determine whether you meet the basic criteria for the study
- Detailed Intake Form
- If you appear to be potentially eligible, our team will send you a more detailed intake form to gather information about your diagnosis and medical history
- Follow-up Call With Our Team
- After reviewing your intake form, a member of our clinical team may contact you to verify your medical history, answer questions, and guide you on next steps
- In-Person Screening Visit
- If you remain eligible you will be scheduled for a screening visit. This includes a review by our providers and any required screening procedures such as laboratory work or medical tests to determine your eligibility