Please fill out the Clinical Trial Inquiry form at the below link for more information.
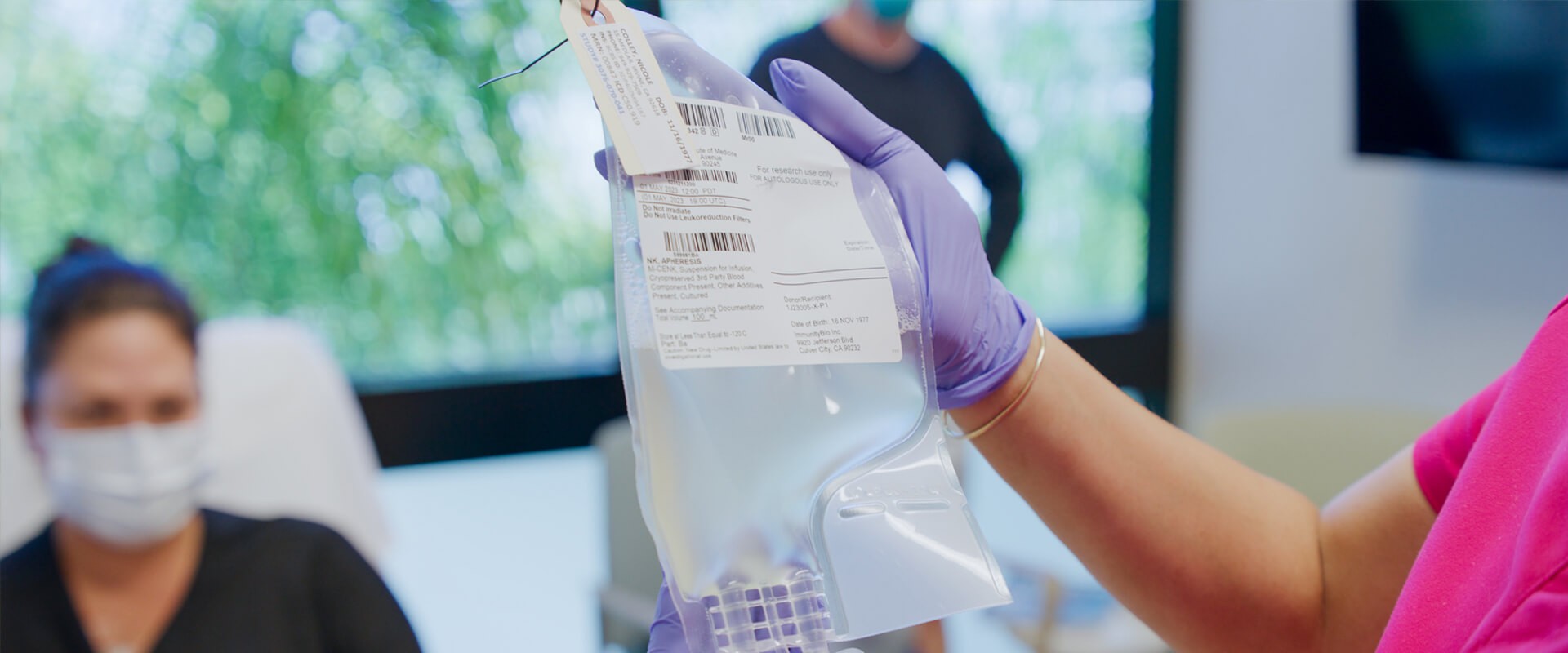



The immune system is a collection of diverse cells, each with a distinct role in protecting us from infections and diseases. Among these are the natural killer (NK) cells, the body’s first line of defense. As part of the ‘innate’ immune response, NK cells have the ability to recognize and destroy abnormal (such as cancerous or virally-infected) cells and are uniquely powerful. They are always activated and ready to eliminate diseased cells without delay. We harness this power with our activated natural killer (aNK) platform based upon NK cells from a distinct cell line.
To further leverage the power of innate immune responses, we have developed a proprietary IL-15 superagonist, Anktiva® (N-803), which selectively activates NK cells and T effector cells, without activating regulatory T cells that may dampen responses. These platforms can be combined for clinical synergy in patients and such combination has produced complete responses in difficult-to-treat cancers, such as Merkel Cell Carcinoma (MCC), advanced pancreatic cancer, and advanced triple negative breast cancer (TNBC).
Our immune-oncology drugs, access to clinical trials, and partnerships with leading research institutions.

Our immune-oncology drugs, access to clinical trials, and partnerships with leading research institutions.

State-of-the-art diagnosis and
treatment options for cancer patients

Our immune-oncology drugs, access to clinical trials, and partnerships with leading research institutions.

Our immune-oncology drugs, access to clinical trials, and partnerships with leading research institutions.
Please complete the form on this page to connect with a CSSIFM research site and start your clinical trial journey
After you submit your info, a CSSIFM staff member will review your info. They will contact you to discuss available clinical trials and/or treatment options.
Depending on the trial, health insurance may be required.
Remember, there is never an obligation to join — we're here to help you make the best decision.
Learn About the Four Clinical Trial Phases
Clinical trials are conducted in a series of steps called “phases.”
Each phase has a different purpose and helps researchers answer different questions.
We begin with a fundamental belief: everyone is born with an innate protective immune system to combat life-threatening diseases such as cancer. Our mission is to unleash that power by orchestrating both the innate and adaptive immune system and change the paradigm in cancer care.


We begin with a fundamental belief: everyone is born with an innate protective immune system to combat life-threatening diseases such as cancer. Our mission is to unleash that power by orchestrating both the innate and adaptive immune system and change the paradigm in cancer care.
Phase 1 trials can take six months to one year to complete.
- The goals of Phase 1 trials are to determine:
- If the investigational medication is safe
- If there are any side effects
- How the investigational medication is broken down by and discharged from the body
- How much investigational medication is needed and how often

Investigational medication is tested for safety on a relatively small group of 20 to 100 volunteers who are usually healthy, but not always.
The trial may happen in a clinic or a hospital.


We begin with a fundamental belief: everyone is born with an innate protective immune system to combat life-threatening diseases such as cancer. Our mission is to unleash that power by orchestrating both the innate and adaptive immune system and change the paradigm in cancer care.
Phase 2 trials can take from six months to one year to complete.
- The goals of Phase 2 trials are to determine:
- How well the investigational medication or vaccine works
- If the investigational medication or vaccine is safe
- If there are any side effects
- How much of the investigational medication or vaccine is needed and how often


We begin with a fundamental belief: everyone is born with an innate protective immune system to combat life-threatening diseases such as cancer. Our mission is to unleash that power by orchestrating both the innate and adaptive immune system and change the paradigm in cancer care.
Phase 3 trials can take one to four years to complete, depending on the disease, length of study, and number of volunteers.
- Researchers closely monitor participants at regular intervals to:
- Confirm that the investigational medication or vaccine is effective
- Identify and monitor side effects
- Compare the medicine or vaccine to commonly used treatments

The investigational medicine or vaccine is tested in approximately 1,000 to 5,000 volunteers.
For medicines, volunteers have the disease or condition the medicine is designed to treat. In vaccine studies, the volunteers may be healthy or have diseases or conditions. Phase 3 trials may happen in a clinic or a hospital.


We begin with a fundamental belief: everyone is born with an innate protective immune system to combat life-threatening diseases such as cancer. Our mission is to unleash that power by orchestrating both the innate and adaptive immune system and change the paradigm in cancer care.
Phase 3 trials can take one to four years to complete, depending on the disease, length of study, and number of volunteers.
- Researchers continue to gather information about:
- The medicine or vaccine and its safety, side effects, and effectiveness
- Marketed products that are studied for new indications

Thousands of people usually participate in ongoing trials.
Phase 4 clinical trials are conducted after the medicine or vaccine has been approved by the appropriate government and regulatory agencies and is being marketed.

More information can be found on clinicaltrials.gov
4:00PM
Clinicaltrials.gov: NCT04390399
Phase: Phase 2

FAQs
QUESTIONS

We begin with a fundamental belief: everyone is born with an innate protective immune system to combat life-threatening diseases such as cancer. Our mission is to unleash that power by orchestrating both the innate and adaptive immune system and change the paradigm in cancer care.

FAQs
A clinical trial is a research study intended to determine if a potential new way to prevent, detect, or treat disease is safe and effective in people with a particular disease or condition. Clinical trials involving volunteer participants are the primary way that researchers evaluate whether a new drug or treatment will work and/or whether it may be more effective, or have fewer side effects, than existing drugs or treatments. According to the U.S. National Institutes of Health (NIH), clinical trials are at the heart of all medical advances and help medical providers and researchers learn more about disease and improve health care for people in the future.

We begin with a fundamental belief: everyone is born with an innate protective immune system to combat life-threatening diseases such as cancer. Our mission is to unleash that power by orchestrating both the innate and adaptive immune system and change the paradigm in cancer care.

FAQs
Clinical trials in most countries are strictly regulated by government agencies. In the U.S., clinical trials are regulated by the Food and Drug Administration (FDA), part of the federal Department of Health and Human Services. Before the FDA approves a clinical trial involving human volunteers, scientists must first perform laboratory tests and studies in animals to evaluate whether a treatment is safe and potentially effective. If these early phase trials show favorable results, the FDA can then approve human clinical trials.
Independent Review Boards (IRBs), which comprise experts in a given therapeutic area, monitor clinical trials to make sure appropriate steps are taken to protect the rights and welfare of trial participants. Under FDA regulations, an IRB has the authority to approve, require modifications in, or disapprove research. Visit the FDA website for more information on how IRBs work.

We begin with a fundamental belief: everyone is born with an innate protective immune system to combat life-threatening diseases such as cancer. Our mission is to unleash that power by orchestrating both the innate and adaptive immune system and change the paradigm in cancer care.

FAQs
Cancer claims millions of lives around the world every year. Cancer researchers are working constantly to identify new treatments for cancer patients that will be better, more effective and/or safer than treatments that exist today. Clinical trials are a vital step in developing those treatments so they can be prescribed to patients and move us ever closer to a cure. Cancer patients who volunteer to participate in a clinical trial are not only advancing research, but also helping ensure new and better treatments are available for other cancer patients.

We begin with a fundamental belief: everyone is born with an innate protective immune system to combat life-threatening diseases such as cancer. Our mission is to unleash that power by orchestrating both the innate and adaptive immune system and change the paradigm in cancer care.

FAQs
People choose to join trials for a variety of reasons. Sometimes they do so because the treatments they have tried for their health problem did not work, or did not produce an acceptable outcome. Other times, there may be no treatment currently available for their health problem. Many trial participants join trials because they want to play a more active role in their own healthcare, or because they want to help researchers learn more about certain health problems. Regardless of their reasons, patients who volunteer to participate in clinical trials may learn about new treatments months or years before they become widely available. The NIH has valuable resources for people considering joining clinical trials.

We begin with a fundamental belief: everyone is born with an innate protective immune system to combat life-threatening diseases such as cancer. Our mission is to unleash that power by orchestrating both the innate and adaptive immune system and change the paradigm in cancer care.

FAQs
Most clinical trials in the United States (and globally) are listed in a database maintained by the National Library of Medicine, and available at the clinicaltrials.gov website. Individuals can search that site by disease or condition, or in other ways, to locate clinical trials of potential interest to them and learn how they or their doctors can contact the researchers in charge of the study. If you are interested in participating in one of ImmunityBio’s clinical trials, it is easy to apply: Simply complete the patient form on our website and a member of our team or a trial partner will follow up with you.

We begin with a fundamental belief: everyone is born with an innate protective immune system to combat life-threatening diseases such as cancer. Our mission is to unleash that power by orchestrating both the innate and adaptive immune system and change the paradigm in cancer care.

State-of-the-art diagnosis and treatment options for cancer patients

2040 East Mariposa Avenue
El Segundo, California 90245
Phone: 213-266-5600
Fax: 562-548-2304

Our immune-oncology drugs, access to clinical trials, and partnerships with leading research institutions.

Our immune-oncology drugs, access to clinical trials, and partnerships with leading research institutions.
1-855-797-9277 | info@CSSIFM.org
© 2024 CSSIFM. All Rights Reserved.
Privacy Policy | Terms of Service | Notice of Privacy Practices